|
Acid (wine), Acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequence of database operations that satisfies the ACID properties (which can be perceived as a single logical operation on the data) is called a ''transaction''. For example, a transfer of funds from one bank account to another, even involving multiple changes such as debiting one account and crediting another, is a single transaction. In 1983, Andreas Reuter and Theo Härder coined the acronym ''ACID'', building on earlier work by Jim Gray who named atomicity, consistency, and durability, but not isolation, when characterizing the transaction concept. These four properties are the major guarantees of the transaction paradigm, which has influenced many aspects of development in database systems. According to Gray and Reuter, the IBM Informa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Computer Science
Computer science is the study of computation, information, and automation. Computer science spans Theoretical computer science, theoretical disciplines (such as algorithms, theory of computation, and information theory) to Applied science, applied disciplines (including the design and implementation of Computer architecture, hardware and Software engineering, software). Algorithms and data structures are central to computer science. The theory of computation concerns abstract models of computation and general classes of computational problem, problems that can be solved using them. The fields of cryptography and computer security involve studying the means for secure communication and preventing security vulnerabilities. Computer graphics (computer science), Computer graphics and computational geometry address the generation of images. Programming language theory considers different ways to describe computational processes, and database theory concerns the management of re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SQL Syntax
The syntax of the SQL programming language is defined and maintained by ISO/IEC SC 32 as part of ''ISO/IEC 9075''. This standard is not freely available. Despite the existence of the standard, SQL code is not completely portable among different database systems without adjustments. Language elements , width=500, caption=A chart showing several of the SQL language elements that compose a single statement. This adds one to the population of the USA in the country table. The SQL language is subdivided into several language elements, including: * ''Keywords'' are words that are defined in the SQL language. They are either reserved (e.g. , and ), or non-reserved (e.g. , and ). List of SQL reserved words. * ''Identifiers'' are names on database objects, like tables, columns and schemas. An identifier may not be equal to a reserved keyword, unless it is a delimited identifier. Delimited identifiers means identifiers enclosed in double quotation marks. They can contain characte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
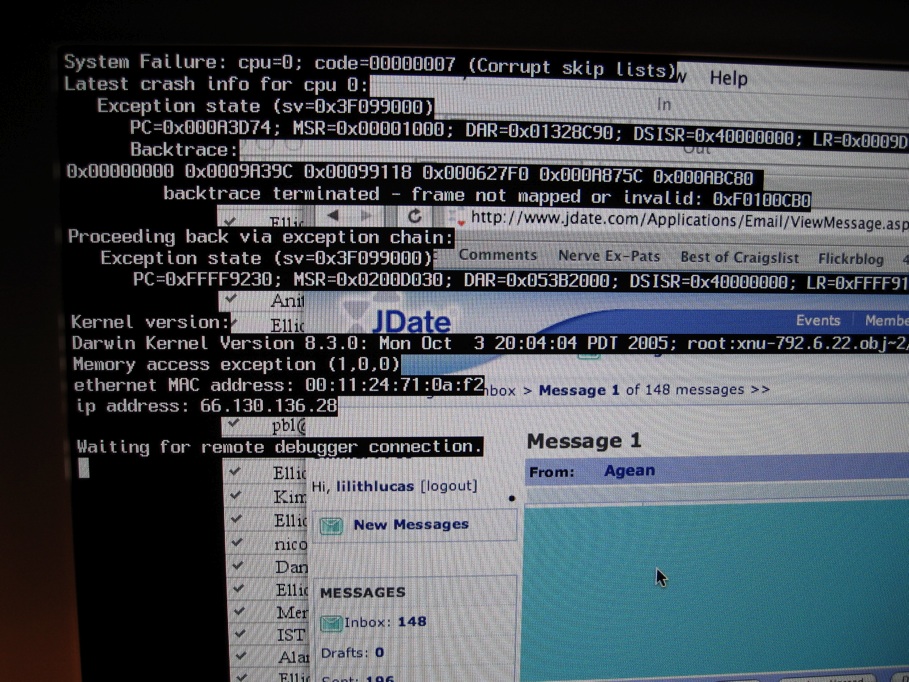
Crash (computing)
In computing, a crash, or system crash, occurs when a computer program such as a software application or an operating system stops functioning properly and exits. On some operating systems or individual applications, a crash reporting service will report the crash and any details relating to it (or give the user the option to do so), usually to the developer(s) of the application. If the program is a critical part of the operating system, the entire system may crash or hang, often resulting in a kernel panic or fatal system error. Most crashes are the result of a software bug. Typical causes include accessing invalid memory addresses, incorrect address values in the program counter, buffer overflow, overwriting a portion of the affected program code due to an earlier bug, executing invalid machine instructions (an illegal or unauthorized opcode), or triggering an unhandled exception. The original software bug that started this chain of events is typically considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Durability (computer Science)
In database systems, durability is the ACID property that guarantees that the effects of transactions that have been committed will survive permanently, even in cases of failures, including incidents and catastrophic events. For example, if a flight booking reports that a seat has successfully been booked, then the seat will remain booked even if the system crashes. Formally, a database system ensures the durability property if it tolerates three types of failures: transaction, system, and media failures. In particular, a transaction fails if its execution is interrupted before all its operations have been processed by the system. These kinds of interruptions can be originated at the transaction level by data-entry errors, operator cancellation, timeout, or application-specific errors, like withdrawing money from a bank account with insufficient funds. At the system level, a failure occurs if the contents of the volatile storage are lost, due, for instance, to system crashes, li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concurrency Control
In information technology and computer science, especially in the fields of computer programming, operating systems, multiprocessors, and databases, concurrency control ensures that correct results for concurrent operations are generated, while getting those results as quickly as possible. Computer systems, both software and hardware, consist of modules, or components. Each component is designed to operate correctly, i.e., to obey or to meet certain consistency rules. When components that operate concurrently interact by messaging or by sharing accessed data (in memory or storage), a certain component's consistency may be violated by another component. The general area of concurrency control provides rules, methods, design methodologies, and theories to maintain the consistency of components operating concurrently while interacting, and thus the consistency and correctness of the whole system. Introducing concurrency control into a system means applying operation constraints ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isolation (database Systems)
In database systems, isolation is one of the ACID ('' Atomicity, Consistency, Isolation, Durability'') transaction properties. It determines how transaction integrity is visible to other users and systems. A lower isolation level increases the ability of many users to access the same data at the same time, but also increases the number of concurrency effects (such as dirty reads or lost updates) users might encounter. Conversely, a higher isolation level reduces the types of concurrency effects that users may encounter, but requires more system resources and increases the chances that one transaction will block another. DBMS concurrency control Concurrency control comprises the underlying mechanisms in a DBMS which handle isolation and guarantee related correctness. It is heavily used by the database and storage engines both to guarantee the correct execution of concurrent transactions, and (via different mechanisms) the correctness of other DBMS processes. The transaction-re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concurrent Computing
Concurrent computing is a form of computing in which several computations are executed '' concurrently''—during overlapping time periods—instead of ''sequentially—''with one completing before the next starts. This is a property of a system—whether a program, computer, or a network—where there is a separate execution point or "thread of control" for each process. A ''concurrent system'' is one where a computation can advance without waiting for all other computations to complete. Concurrent computing is a form of modular programming. In its paradigm an overall computation is factored into subcomputations that may be executed concurrently. Pioneers in the field of concurrent computing include Edsger Dijkstra, Per Brinch Hansen, and C.A.R. Hoare. Introduction The concept of concurrent computing is frequently confused with the related but distinct concept of parallel computing, Pike, Rob (2012-01-11). "Concurrency is not Parallelism". ''Waza conference'', 11 Ja ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Foreign Key
A foreign key is a set of attributes in a table that refers to the primary key of another table, linking these two tables. In the context of relational databases, a foreign key is subject to an inclusion dependency constraint that the tuples consisting of the foreign key attributes in one relation, R, must also exist in some other (not necessarily distinct) relation, S; furthermore that those attributes must also be a candidate key in S. In other words, a foreign key is a set of attributes that a candidate key. For example, a table called TEAM may have an attribute, MEMBER_NAME, which is a foreign key referencing a candidate key, PERSON_NAME, in the PERSON table. Since MEMBER_NAME is a foreign key, any value existing as the name of a member in TEAM must also exist as a person's name in the PERSON table; in other words, every member of a TEAM is also a PERSON. Summary The table containing the foreign key is called the child table, and the table containing the candidate ke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unique Key
In relational database management systems, a unique key is a candidate key. All the candidate keys of a relation can uniquely identify the records of the relation, but only one of them is used as the primary key of the relation. The remaining candidate keys are called unique keys because they can uniquely identify a record in a relation. Unique keys can consist of multiple columns. Unique keys are also called alternate keys. Unique keys are an alternative to the primary key of the relation. In SQL, the unique keys have a UNIQUE constraint assigned to them in order to prevent duplicates (a duplicate entry is not valid in a unique column). Alternate keys may be used like the primary key when doing a single-table select or when filtering in a ''where'' clause, but are not typically used to join multiple tables. Summary Keys provide the means for database users and application software to identify, access and update information in a database table. ''There may be several keys in any ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Referential Integrity
Referential integrity is a property of data stating that all its references are valid. In the context of relational databases, it requires that if a value of one attribute (column) of a relation (table) references a value of another attribute (either in the same or a different relation), then the referenced value must exist. For referential integrity to hold in a relational database, any column in a base table that is declared a foreign key can only contain either null values or values from a parent table's primary key or a candidate key. In other words, when a foreign key value is used it must reference a valid, existing primary key in the parent table. For instance, deleting a record that contains a value referred to by a foreign key in another table would break referential integrity. Some relational database management systems (RDBMS) can enforce referential integrity, normally either by deleting the foreign key rows as well to maintain integrity, or by returning an error and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Database Trigger
A database trigger is procedural code that is automatically executed in response to certain Event (computing), events on a particular Table (database), table or View (database), view in a database. The trigger is mostly used for maintaining the Database integrity, integrity of the information on the database. For example, when a new record (representing a new worker) is added to the employees table, new records should also be created in the tables of the taxes, vacations and salaries. Triggers can also be used to log historical data, for example to keep track of employees' previous salaries. Triggers in DBMS Below follows a series of descriptions of how some popular DBMS support triggers. Oracle In addition to triggers that fire (and execute PL/SQL code) when data is modified, Oracle Database, Oracle 10g supports triggers that fire when schema-level objects (that is, tables) are modified and when user logon or logoff events occur. Schema-level triggers * After Creation * Bef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cascading Rollback
In database technologies, a rollback is an operation which returns the database to some previous state. Rollbacks are important for database integrity, because they mean that the database can be restored to a clean copy even after erroneous operations are performed. They are crucial for recovering from database server crashes; by rolling back any transaction which was active at the time of the crash, the database is restored to a consistent state. The rollback feature is usually implemented with a transaction log, but can also be implemented via multiversion concurrency control. Cascading rollback A cascading rollback occurs in database systems when a transaction (T1) causes a failure and a rollback must be performed. Other transactions dependent on T1's actions must also be rollbacked due to T1's failure, thus causing a cascading effect. That is, one transaction's failure causes many to fail. Practical database recovery techniques guarantee cascadeless rollback, therefore a casc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |